Abstract
Background: Single agent ibrutinib is currently approved as monotherapy for the treatment of CLL, both in treatment-naïve and relapsed patients; it induces high response rates, which are durable in most patients. Results from a Phase II study combining ibrutinib with rituximab in high-risk patients with CLL demonstrated encouraging high overall response rates (ORR 95%, Burger JA, Lancet Oncology 14: 1090, 2014). To determine whether rituximab provides added benefit to ibrutinib therapy, we conducted an open label randomized single center trial of ibrutinib versus ibrutinib plus rituximab in 206 patients with CLL (NCT02007044).
Methods: Patients were randomized to receive ibrutinib (Ib, n= 102) or ibrutinib plus rituximab (Ib+R, n= 104). Patients with relapsed CLL and treatment naïve patients with high-risk disease (del17p or TP53 mutation, n=27), who were evenly distributed among the treatment arms, were eligible. Patients were treated with ibrutinib 420 mg PO daily, until adverse events, disease progression, or death precluded further therapy. Patients randomized to Ib+R received rituximab during the first 6 months of treatment (375 mg/m2 weekly during the first 4 weeks [cycle 1], then monthly for cycles 2-6), in addition to ibrutinib. The primary end point was progression free survival (PFS); secondary end points included ORR per revised iwCLL criteria, safety, and tolerability.
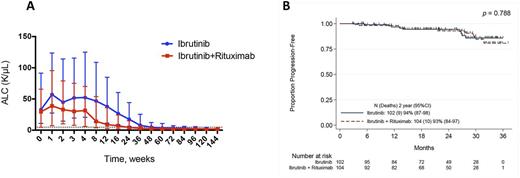
Results: The median age was 65 years, 70% were male, 37% had del17p or TP53 mutation, 20% del11q, 72% unmutated IgHV, and 38% advanced stage disease (Rai stage 3-4). The median baseline absolute lymphocytic count (ALC) and β2 microglobulin at start of therapy were 30x109/L (0.5 - 350.9x109/L) and 3.7 mg/L (1.3 - 13.1 mg/L), respectively. After a median observation time of 25.2 and 22.7 months, 79 (77%) or 71 (68%) of patients continue ibrutinib treatment on the Ib or Ib+R arm, respectively. 188 patients were evaluable for response assessment. 20 patients (21%) on the Ib arm and 26 patients (28%) on the Ib+R arm achieved a complete remission (CR), p=0.309; partial remissions (PR) were achieved in 72 (77%) and 68 (72%) of patients receiving Ib or Ib+R, respectively, accounting for an ORR of 98% for Ib-, and 100% for Ib+R-treated patients. Bone marrow flow cytometry assessments for minimal residual disease (MRD) at time of last follow up showed significantly lower level of residual CLL cells in Ib+R treated patients (median: 4.9% CLL cells) when compared to Ib treated patients (17.1%, p=0.002). CR with MRD-negativity was observed in 5 patients treated with Ib+R and in 1 patient treated with Ib. The median time to normalization of the absolute lymphocyte count (ALC, ≤4.0 K/uL) was significantly shorter in Ib+R- compared to Ib-treated patients (3.0 vs. 8.9 months, p<0.001) (Figure 1A). The median time to achieve CR was also significantly shorter in Ib+R treated patients (11.5 months, versus 21.1 months in the Ib treated patients; p=0.032); however, there was not a significant difference in PFS between the patients treated with Ib and Ib+R during the observation period of time (91.2% vs. 90.4%, p=0.788, Figure 1B). Among the 56 patients that came off study (23 from Ib, and 33 from Ib+R), side effects and/or toxicities were the most common cause for therapy discontinuation (n=28); death was reported in 5 patients. Causes of death were renal failure, cerebral hemorrhage, bowel perforation with colon hematoma, pneumonia, and respiratory failure. 8 patients had disease progression (5 on Ib and 3 on the Ib+R arm); disease transformation was reported in 3 patients, occurring between 11 to 16 months on treatment. Discontinuation due to second malignancies was reported in 6 patients with colorectal cancer, liposarcoma, melanoma, pleomorphic sarcomatoid tumor, and 2 cases of new-onset CML. The most frequently reported related adverse events (AE) were similarly distributed in both arms, including arterial hypertension, neutropenia, diarrhea, and atrial fibrillation.
Conclusion: The addition of rituximab to ibrutinib in relapsed and high-risk CLL patients did not improve the PFS. However, patients treated with Ib+R reached their remissions significantly faster and achieved lower MRD levels. Given these results, single-agent ibrutinib should remain standard-of-care therapy in CLL, but the addition of rituximab can be considered in patients in whom a faster response is desirable.
Burger: TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Jain: Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Incyte: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; BMS: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Verastem: Research Funding. Kantarjian: Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; Delta-Fly Pharma: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; ARIAD: Research Funding. O'Brien: Acerta: Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; GSK: Consultancy; Vaniam Group LLC: Consultancy; Astellas: Consultancy; Aptose Biosciences, Inc.: Consultancy; Alexion: Consultancy; Pfizer: Consultancy, Research Funding; Celgene: Consultancy; Sunesis: Consultancy; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; AbbVie: Consultancy; Amgen: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Janssen: Consultancy; CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Regeneron: Other: Research Support: Honorarium, Research Funding. Wierda: Kite: Research Funding; Juno: Research Funding; The University of Texas MD Anderson Cancer Center: Employment; Sanofi: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding; GSK/Novartis: Consultancy, Honoraria, Research Funding; Genzyme: Consultancy, Honoraria; Merck: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Emergent: Consultancy, Honoraria, Research Funding; Karyopharm: Research Funding; Acerta: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal